
Journal of the Bahrain Medical Society
Year 2017, Volume 29, Issue 3, Pages 13-18
https://doi.org/10.26715/jbms.29.3.2017.38aShamaila Dar1*, Manaf Al Qahtani1, Fajer Altamimi1
1Department of Medicine, Bahrain Defence Forces Hospital, P.O. Box-28743, West Riffa, Kingdom of Bahrain.
*Corresponding author
Dr. Shamaila Dar, Department of Medicine, Bahrain Defence Forces Hospital, P.O. Box-28743, West Riffa, Kingdom of Bahrain, Email: shamaila.imran@bdfmedical.org
Received date: December 22, 2016; Accepted date: August 9, 2017; Published date: August 18, 2017

The association between the occurrence of influenza and cryptogenic organizing pneumonia is very important in the clinical practice. In this case report, we presented a 58-year-old woman who came to seek medical advice for fever, abdominal pain, and diarrhea. A few days later, she got desaturated and was diagnosed with influenza A. Computed tomography of the chest showed interstitial pneumonitis symptoms. This case study highlights the role of glucocorticoids in improving the outcome of such patient.
Introduction
Influenza is an acute respiratory illness caused by influenza A or B virus. It often occurs worldwide, usually in the winter season. Infection can be transmitted through large-particle droplets. Influenza characteristically starts with a sudden onset of fever, headache, myalgia, and malaise after an incubation period of 1-4 days. Patient may experience a nonproductive cough, sore throat, and nasal discharge.1
One of the major complications of influenza is pneumonia, which occurs most often in certain groups of patients having underlying chronic illnesses who are classified as high risk. Such patients include children <2 and individuals>65 years of age; patients with diabetes, asthma, and immunosuppression; pregnant women, and those living in nursing homes.1
The types of pneumonia that are encountered are categorized as primary viral pneumonia, secondary bacterial pneumonia, or a mixture of both. Central nervous system disease may be associated with influenza; manifestations may include encephalopathy, encephalitis, transverse myelitis, aseptic meningitis, and Guillain-Barre syndrome. Other complications of influenza include myositis, rhabdomyolysis, myocarditis, pericarditis, and toxic shock syndrome.1
Previous literature has cited the use of steroids in the treatment of influenza-associated cryptogenic organizing pneumonia (COP).2 COP (formerly called bronchiolitis obliterans organizing pneumonia or BOOP) is a form of diffuse interstitial lung disease, which affects the distal bronchioles, respiratory bronchioles, alveolar ducts, and alveolar walls. The primary area of injury is within the alveolar wall.3-6
COP typically occurs in the fifth or sixth decades of life and affects men and women equally. Most patients are usually symptomatic for less than 2 months and have similar symptoms to that of community-acquired pneumonia (cough, dyspnea with exertion, and weight loss).7 Approximately half of the cases are indicated by a flu-like illness.4, 8, 9 Nonspecific interstitial pneumonia can be idiopathic or may be associated with connective tissue disease, certain drugs, human immunodeficiency virus infection, and hypersensitivity pneumonitis.10, 11 The most common chest imaging features of COP are multiple ground-glass or consolidative opacities.12-16 Pulmonary function tests usually show a restrictive pattern with an associated gas transfer defect. Corticosteroids are used in the treatment of patients with moderate to severe respiratory impairment. Clinical manifestations improve within 48 h with corticosteroids in symptomatic patients. Radiographic pulmonary infiltrates usually disappear within few weeks.
Case presentation
A 58-year-old woman with known case of diabetes mellitus, dyslipidemia, and hypothyroidism was admitted with a history of fever for 4 days, abdominal pain, and diarrhea for 1 day. The diarrhea was loose watery, without mucus or blood for the past 1 day, 4-5 times/day. There was no history of ingestion of unpasteurized milk, and recent travel; however, there was a history of sick contact at work. No history of dyspnoea, cough or sputum, sore throat or flu like symptoms were observed. The fever was high grade and had not responded to oral antibiotic—Amoxiclav (amoxicillin+clavulanic acid). Initial impression was that of tonsillitis and to rule out brucellosis.
Medical history: Diabetes mellitus for 1 year; hypertension and dyslipidemia for approximately 5 years.
Medication history: Glimepiride 3 mg once a day.
Social history: Married, did not have children, nonsmoker, and nonalcoholic. She was working in the Ministry of Education.
Family history: She had a family history of diabetes mellitus, hypertension, dyslipidemia, and ischemic heart disease.
Physical examination: Vitals on admission: Blood pressure, 153/60 mmHg; pulse, 73 beats/ min; random blood glucose, 15 mmol/L; oxygen saturation in room air, 100%; and respiratory rate, 18 breaths/min.
Examination of throat: Mild congestion
Chest: Normal vesicular breathing, no added sounds.
The rest of the physical examination was unremarkable.
Table 1: Investigations and patient values.
ALT, Alanine transaminase; AST, Aspartate aminotransferase; ESR, Erythrocyte sedimentation rate; Anti EBV, Anti Epstein–Barr virus; IgG, Immunoglobulin G; IgM, Immunoglobulin M; HBsAg, Hepatitis B virus surface antigen; HCV, Hepatitis C virus; HIV Ag/Ab Combo, Human immunodeficiency virus antigen/antibody combination; ANA and Anti-dsDNA, Antinuclear antibodies and anti-double stranded DNA; HbA1c, Hemoglobin A1c (Glycated hemoglobin) Ultrasound scan (Abdomen): Mild diffuse fatty
infiltration.
Computed tomography pulmonary angiography: The investigation was negative for pulmonary embolism. Bilateral peripherally located subpleural wedge-shaped patches of ground-glass appearance with early consolidation. It was more apparent in relation to the basal surfaces of both lung lobes and were seen centered on the hilum. A small emphysematous bulla was seen on the left lower lung lobe pleural surface. There was no evidence of significant large pulmonary nodules or masses, which could be due to underlying atypical pneumonia.
Echocardiography: Unremarkable
Clinical course
The patient was admitted with the impression of tonsillitis and was started treatment with antibiotics, as she had not responded to Amoxiclav (amoxicillin+clavulanic acid). Patient spiked fever on the fourth day and desaturated to 90% on room air. The differential diagnosis was infective pneumonitis that is, viral, bacterial, rickettsia, fungal, chronic organizing pneumonia, sarcoidosis, alveolar proteinosis, Loffler’s pneumonia, pulmonary embolism (in view of travel by air for 12 h a week back). Figure 1, 2, and 3 represent the chest x-ray on day 1, 4, and 8, respectively. Figure 4 represents the chest x-ray after treatment with glucocorticoids.
Computed tomography pulmonary angiography was performed to rule out pulmonary embolism, which showed bilateral patchy consolidation with a predominantly subpleural distribution as shown in the Figures 5a, b, and c. The final diagnosis was viral pneumonitis secondary to influenza A virus.

Figure 1: Plain x-ray chest (Posteroanterior (PA) view) day 1: Bilateral accentuated bronchovascular marking with no significant consolidation, pulmonary nodule or mass. No pleural effusion

Figure 2: Plain x-ray chest (PA view) day 4 of admission: Bilateral ill-defined patchy peripheral, subpleural and peribronchovascular areas of consolidation affecting all the lung zones with partial obliteration of both costophrenic angles.
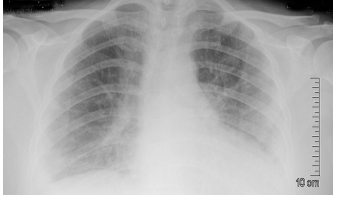
Figure 3: Plain x-ray chest (PA view) day 8 of admission: More conspicuous appearance of the peripheral consolidation patches with obliteration of both costophrenic angles and silhouetting the left diaphragmatic cupola
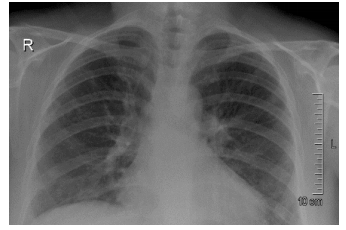
Figure 4: Plain x-ray chest (PA view) after treatment with glucocorticoids showing dramatic improvement in the clearance of both costophrenic angles and the peripheral consolidation patches, especially on the left side
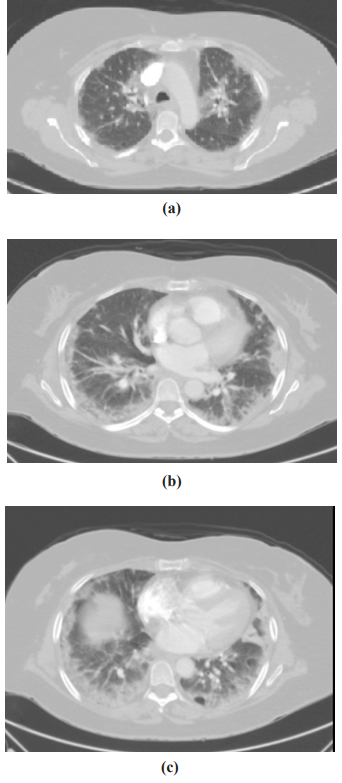
Figure 5: Postcontrast axial computed tomography of the chest (pulmonary window) through the upper (a), middle, (b) and lower (c) lung lobes: Evidence of bilateral patchy consolidation with a predominantly subpleural distribution associated with peribronchial cuffing and linear opacities with an arcade or polygonal appearance
Treatment
The patient was kept initially on ceftriaxone 1 gm (intravenous) once daily and ciprofloxacin 400 mg (orally) twice a day, which was later changed to tazobactam/piperacillin 4.5 gm (intravenous) every 8 h and clarithromycin (modified-release preparation) 500 mg (orally) once daily in the view of suspected bacterial pneumonia.
Oseltamivir 75 mg (orally) was administered twice daily for 10 days to cover viral pneumonia. The blood pressure on admission was 153/60 mmHg, which on later days was in the range of 125-130/60-65 mmHg; so no treatment was initiated. NovoMix-30 (Insulin aspart) FlexPen (subcutaneous) was used twice a day to control the hyperglycemia. Prednisolone was planned to be kept at 1 mg/kg; however, patient insisted to keep a lower dose and quicker tapering dose in view of uncontrolled blood glucose. Therefore, prednisolone 50 mg (orally) once daily was prescribed and 10 mg (orally) every week was instituted as a tapering dose. Figure 4 shows improvement in chest x-ray after steroid treatment. The patient was discharged after 10 days of hospital admission, in a vitally stable and satisfactory condition.
Follow-up
The patient was consulted with infectious disease clinic and had a great response to steroids with the clearing of the pneumonitis using radiology; however, the steroids were tapered off over 5 weeks. On follow-up with the endocrinologist, the blood glucose levels were reviewed and insulin doses were adjusted accordingly.
Discussion
Severe Influenza A infection may result in COP in some cases for which steroids have been successfully used. In our patient, the computed tomography of the chest showed evidence of bilateral patchy consolidation with a predominantly subpleural distribution associated with peribronchial cuffing and linear opacities with an arcade or polygonal appearance. Although the mechanisms and pathogenesis of these cases are not fully understood, acute diffuse alveolar damage by neutrophils on the alveolar wall was found by radiological assessment. Based on these findings, several case reports have demonstrated about steroid response.1
Hetrogenenicity of published data regarding the study design, severity of illness, dosing, and type of corticosteroid administered constitute an important limitation for drawing a robust conclusion. Recent insights on the benefits of corticosteroids had been shown in few case reports. In patients with acute respiratory distress syndrome, with and without confirmed influenza A infection, prolonged lowto-moderate dose corticosteroid treatment was well tolerated and associated with significant improvement in lung injury and multiple organ dysfunction scores and a low hospital mortality.17-20
The decision to initiate the therapy for COP and the choice of initial therapy depends on multiple factors such as severity of symptoms, pulmonary function impairment, the radiographic extent of disease, and the rapidity of progression. It is reasonable to monitor COP patients without therapy if they are minimally symptomatic with either mild or absence of abnormalities in pulmonary function tests. Patients should be reassessed at 8-12 weeks intervals regarding any increase in symptoms, worsening of pulmonary functions, or progression of the radiographic opacities.
For symptomatic patients with moderate or more severe respiratory impairment due to COP, initiation of systemic glucocorticoid therapy is recommended. The usual dose is equivalent of prednisolone 0.75-1 mg/kg/day (using ideal body weight) to a maximum of 100 mg/day given as a single oral dose in the morning; most patients respond to a dose of 60 mg per day. For patients with the rapidly progressive disease or impending respiratory failure, an initial high-dose glucocorticoid therapy is suggested with methylprednisolone 500-1000 mg intravenously for 3–5 days rather than a lower oral dose. For patients who fail to respond to an initial therapy with systemic glucocorticoids or presented with the fulminant disease, an addition of a second immunosuppressive agent is recommended, such as cyclophosphamide and azathioprine.19, 20
Glucocorticoid therapy-induced clinical improvement and clearing of the patchy opacities on the chest imaging usually takes several days to few weeks. Oral glucocorticoid therapy is slowly tapered, as tolerated by the patient over 6-12 months. Relapses are common upon stopping or reduction of glucocorticoids, often leading to prolonged treatment. For patients who are unable to taper glucocorticoids to a level that does not cause intolerable adverse effects, a second immunosuppressive agent, such as azathioprine, is added.20
It is reasonable that steroids have demonstrated a beneficial effect in the treatment of patients with severe influenza pneumonitis infection. Moreover, it is shown that corticosteroids might be associated with less duration of invasive ventilation and intensive care unit stay.
Conclusion
Our patient had early detection of influenza A and quick response to corticosteroids. It was a valuable experience in raising awareness among physicians to this particular diagnosis. It is warranted to ensure providing proper and timely treatment impact the patient’s outcome. Thus glucocorticoids use in influenza pneumonitis should be preferred in selected cases, such as our patient.
1. Kudo K, Takasaki J, Manabe T, et al. Systemic corticosteroids and early administration of antiviral agents for pneumonia with acute wheezing due to influenza A (H1N1) pdm09 in Japan. PloS one. 2012;7(2):e32280.
2. .Mollura DJ, Asnis DS, Crupi RS, et al. Imaging findings in a fatal case of pandemic swineorigin influenza A (H1N1). Am J Roentgenol. 2009;193(6):1500-3.
3. Sara A-G, Hamdan A-J, Hanaa B, et al. Bronchiolitis obliterans organizing pneumonia: pathogenesis, clinical features, imaging and therapy review. Ann Thorac Med. 2008;3(2):67.
4. Cordier J. Cryptogenic organising pneumonia. Eur Respir J. 2006;28(2):422-46.
5. Epler GR. Bronchiolitis obliterans organizing pneumonia. Arch Intern Med. 2001;161(2):158-64.
6. Cordier J-F. Organising pneumonia. Thorax. 2000;55(4):318-28.
7. Greenberg-Wolff I, Konen E, Dov IB, et al. Cryptogenic organizing pneumonia: variety of radiologic findings. IMAJ-RAMAT GAN-.2005;7(9):568.
8. Montesinos JJ, Laguna MA. Case 1: Cryptogenic organizing pneumonia. AJR Am J Roentgenol. 1998;171(3):835, 8-9.
9. Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial .pneumonias. Eur Respiratory Soc; 2002.
10. Kim SJ, Lee KS, Ryu YH, et al. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. Am J Roentgenol. 2003;180(5):1251-4.
11. Webb WR, Muller NL, Naidich DP. Highresolution CT of the lung: Lippincott Williams & Wilkins; 2014.
12. McQueen AS, Grainger RG, Grant LA, et al. Grainger & Allison’s Diagnostic Radiology: Single Best Answer MCQs: Elsevier Health Sciences; 2009.
13. Lee KS, Kullnig P, Hartman TE, et al. Cryptogenic organizing pneumonia: CT findings in 43 patients. AJR Am J Roentgenol. 1994;162(3):543-6.
14. Wittram C, Mark EJ, McLoud TC. CT-histologic correlation of the ATS/ERS 2002 classification of idiopathic interstitial pneumonias Radiographics. 2003;23(5):1057-71.
15. Mueller-Mang C, Grosse C, Schmid K, et al. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27(3):595-615.
16. Lynch DA, Travis WD, Muller NL, et al. Idiopathic interstitial pneumonias: CT features. Radiology. 2005;236(1):10-21.
17. Quispe-Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36(1):33-41.
18. Ariani F, Liu K, Jing Z, et al. Glucocorticosteroid in treatment of severe pneumonia. Mediators Inflamm. 2013;2013:8.
19. Yousem SA, Lohr RH, Colby TV. Idiopathic bronchiolitis obliterans organizing pneumonia/cryptogenic organizing pneumonia with unfavorable outcome: pathologic predictors. Mod Pathol. 1997;10(9):864-71.
20. Vaz A, Morais A, Melo N, et al. Azithromycin as an adjuvant therapy in cryptogenic organizing pneumonia. Rev Port Pneumol. 2011;17(4): 186-9.