
Journal of the Bahrain Medical Society
Year 2014, Volume 25, Issue 1, Pages 33-41
https://doi.org/10.26715/jbms.p25_8Zainab Abbas Al Hasan
Consultant Family Physician, Hamad Kanoo Health Center, Bahrain
Correspondence to: ZHasan5@health.gov.bh

Objective: To investigate the prevalence of albuminuria (microalbuminuria and macroalbuminuria) in patients with type 2 diabetes mellitus (DM) attending the National Center of Diabetes, Endocrinology, and Genetics (NCDEG) in Jordan. The second objective of the study was to determine if there is any association between albuminuria (microalbuminuria and macroalbuminuria) and a number of independent variables, including glycemic control, hypertension, duration of diabetes mellitus, serum lipids, body mass index, smoking, age, gender and the presence of retinopathy.
Methods: A cross-sectional study was carried out at the National Center for Diabetes, Endocrinology, and Genetics (NCDEG), Jordan. A total of 869 diabetic patients aged 30 years and above with type 2 diabetes mellitus who attended the clinic from 1st September 2007 through 10th January 2008 were included. At least two out of three random morning spot urine samples were collected in a three-month period to determine the urinary albumin-creatinine ratio (ACR). Microalbuminuria is defined as an ACR of 30-299 mg/g and macroalbuminuria is defined as an ACR >300 mg/g according to the definition proposed by the American Diabetes Association (ADA). Socio-demographic, clinical, and laboratory data were obtained from the medical records of patients. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS, version 11.5).
Results: Out of the 880 patients recruited, 5 (0.6%) were excluded due to the presence of urinary tract infections and 6 (0.7%) for the presence of congestive heart failure, leaving 869 patients for evaluation. There were 56.0% male and44% female. The mean age and duration of diabetes were 57.8 years and 9.5 years, respectively. Approximately 62% were being managed by oral hypoglycemic agents alone, 4.3% by insulin alone, 31.7% were on a combination of oral hypoglycemic agents and insulin and slightly less than 2% were on dietary measures alone. The mean value for HbA1c was 7.71%. The overall prevalence of albuminuria among participants was found to be 34.6%; microalbuminuria (29.3%) and macroalbuminuria (5.3%). Multivariate logistic regression analysis identified HbA1c, SBP, DBP and the presence of retinopathy as independent risk factors for microalbuminuria and SBP. Male gender and presence of retinopathy were identified as independent risk factors for macroalbuminuria.
Conclusion: Albuminuria is highly prevalent among Jordanians with type 2 diabetes. This calls for early and universal screening of urinary albumin. There is also an urgent need for measures that target tight glycemic and optimal blood pressure control and the use of renin-angiotensin system blockade.
Keywords: diabetes mellitus; microalbuminuria; macroalbuminuria; Jordan
Diabetes mellitus (DM) is considered to be a major health problem that is predicted to turn into a global epidemic, affecting around 171 million people worldwide. The World Health Organization predicts that this number will rise to 366 million by 20301. In Jordan, the reported prevalence of diabetes among adults was 13.4% in 1998, while in a recent study the age standardized prevalence of DM was 17.1%; confirming that the prevalence of DM in Jordan is also increasing2.
Among all the complications of diabetes mellitus, nephropathy is the diabetes-specific complication with the
greatest mortality3. Although in the past the risk of renal complications were thought to be considerably lower among patients with type 2 diabetes than among those with type 1 diabetes3, there is an increasing body of evidence that the risk of nephropathy with progression to end-stage renal disease (ESRD) is not dissimilar in the two groups4.
It is not surprising to see the emergence of more patients with ESRD among type 2 diabetics because of the increasing prevalence of type 2 diabetes and the improved survival rates of diabetic patients with renal failure4. Nephropathy associated with type 2 diabetes is the most frequent cause of ESRD in the United States, Europe, and Japan3. In Jordan, DM was the cause of ESRD in 10.5% of patients on haemodialysis in 19925, increasing to 29.2% in 20036. Although the magnitude of the disease has been better defined in developed countries, the situation is particularly serious in the developing countries where healthcare resources may often be limited7. One of the initial markers of this condition is microalbuminuria, which generally indicates an increased risk of deteriorating renal function and mortality8. Microalbuminuria is also a well-established marker of increased cardiovascular disease risk8. It is defined as 30 to 299 mg/dl in a 24-hr collection or 30 to 299 μg/mg in a spot collection, whereas macroalbuminuria is defined as ≥300 mg/dl in a 24-hr collection or ≥300 μg/mg in a spot collection9. Without specific interventions, 20-40% of type 2 diabetic patients with microalbuminuria progress to macroalbuminuria (overt nephropathy), but by 20 years after the onset of overt nephropathy, only ~20% will have progressed to end-stage renal disease9. Macroalbuminuria is a strong and independent predictor of increased cardiovascular morbidity and mortality and its presence predicts loss of kidney function10. Diabetic retinopathy can be found in more than 90% of type 1 diabetic patients with nephropathy11. However, the relationship between albuminuria and diabetic retinopathy (DR) was less predictable in type 2 diabetes11.
Several studies have shown that there is a significant association between diabetic nephropathy and variables such as glycemic control, hypertension, hyperlipidemia, smoking, obesity, and duration of DM 8, 9, 11, 12, 13, 14.
A better understanding of the prevalence of albuminuria and the associated risk factors is required to enable the further development of strategies for diabetic nephropathy in Jordanian patients diagnosed with type 2 diabetes, in view of the increased prevalence of diabetes and IFG in Jordan 2. To date, there is no data available documenting the prevalence of albuminuria among type 2 diabetics in Jordan.
A cross-sectional study was conducted at the National Center for Diabetes, Endocrinology and Genetics (NCDEG) in Amman, Jordan. It comprised a systematic sample of 869 type 2 diabetic patients attending the NCDEG from 1st September 2007 through 10th January 2008. The study was approved by the NCDEG Ethics Committee. Relevant sociodemographic, clinical and laboratory data were obtained from the medical records of the patients. Anthropometric measurements were taken, including weight and height to estimate body mass index (BMI) [Kg/m2]. ‘Overweight’ is classified as having a BMI of 25.0 to 29.9, and obesity as having a BMI of ≥30 as defined by the WHO15. Blood pressure was measured using standardized sphygmomanometers on the right upper arm while the subject was in a sitting position after 5 minutes’ rest. Fasting blood sugar, serum cholesterol, serum triglyceride, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were assayed by enzymatic methods. Glycosylated hemoglobin (HbA1c) was analyzed by using a highperformance liquid chromatography (HPLC) method (Bio-Rad); HbA1c was considered normal if <7%16.
Fresh random morning spot urine was examined by the strip test (Combur 9 Test) for leucocytes, nitrites and blood. Patients with positive nitrites, indicative of significant bacteriuria, and patients with erythrocytes and/ or leucocytes equal to or above 5 cell counts per microliter, indicative of hematuria or/and pyuria, were excluded from the analyses. Microalbuminuria can be diagnosed when the urinary albumin to creatinine ratio is 30-299 mg/g in at least two of three urine collections in a three-month period. Whereas to designate a patient as having macroalbuminuria the same ratio is ≥300 mg/g 9.
Diabetic retinopathy was detected by direct ophthalmoscopy. The International Clinical DR severity scale, adopted by the American Academy of Ophthalmology (AAO)17 and the International Council of Ophthalmology (ICO)18, was used to classify patients into non-proliferative DR (NPDR), and proliferative diabetic retinopathy (PDR).
Statistical analysis was carried out using Statistical Package for the Social Sciences (SPSS, version 11.5). Chi-square tests and multivariate logistic regression was used to assess the correlation between microalbuminuria and macroalbuminuria and their risk factors. Separate regression models were used for microalbuminuria and macroalbuminuria.
Of the 880 patients recruited, 5 (0.6%) were excluded due to the presence of urinary tract infections and 6 (0.7%) for the presence of congestive heart failure, leaving 869 patients for evaluation. There were 487 (56%) men and 382 (44%) women. The mean age of the total study population was 57.8 ±9.1 years and the mean duration of diabetes was 9.5 ±7.5 years. Of the total sample, 15.9% were smokers while 20.7% were past smokers and 90.4% were obese or overweight. The majority of the patients had systolic and diastolic blood pressure below the 130/80 mm Hg target (see Table 1).
As shown in Table 2, the majority of the sample population (67.9%) had hypertension. Dyslipidemia was present in 94.6% of the study patients. Hypertriglyceridemia, hypercholesterolemia, high LDL cholesterol, low HDL cholesterol levels among males and low HDL cholesterol levels among females were reported among 40.4%, 13.5%, 46.1%, 46.4%, 58.9% of these patients, respectively. Only 35.3% had HbA1c <7%. About one-third of patients had retinopathy.
Table 1. Sociodemographic and anthropometric characteristics
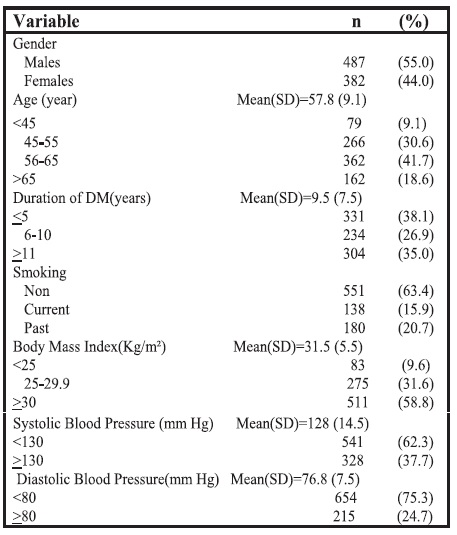
Table 2. Clinical and biochemical characteristics of the participants
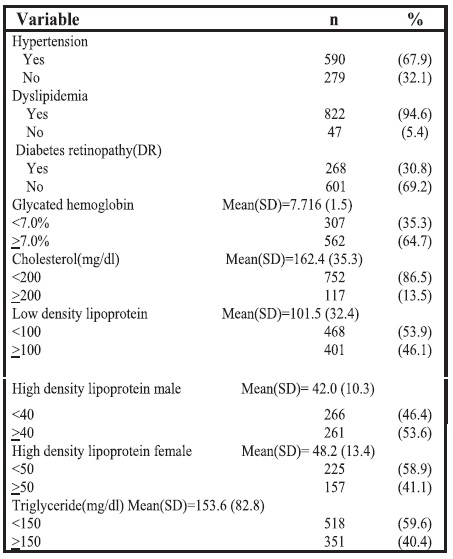
A high proportion of the diabetic patients (62.4%) were managed by oral medication alone, 4.3% by insulin alone, 31.7% of the patients were on a combination of oral medication and insulin, while 1.6% of the patients were following only a dietary regimen as part of their diabetes management.
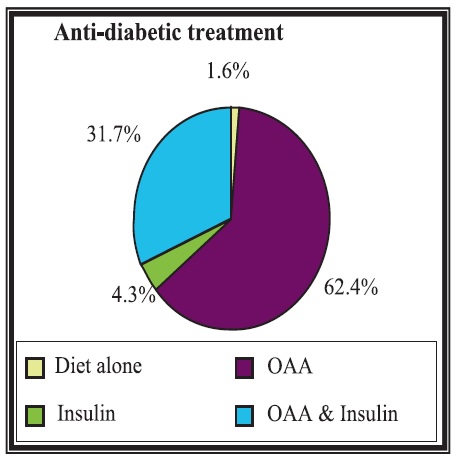
Figure 1. Anti-diabetic treatment; OAA: Oral Anti-diabetic Agents
Most of the study participants (74.8%) were also on antihypertensive treatment. Drugs used were angiotensinconverting enzyme inhibitors (ACE) (38.1%), angiotensin receptor blockers (ARBs) (27.5%), combined ACEI and ARBs (1%), beta-blockers (32.1%), alpha-blockers (1.4%), dihydropyridine calcium channel blockers (18.9%), nondihydropyridine ca-channel blockers (3.5%) and diuretic (28.7%) (see Figure 2). The majority of patients (74.5%) were receiving a lipid-lowering agent.
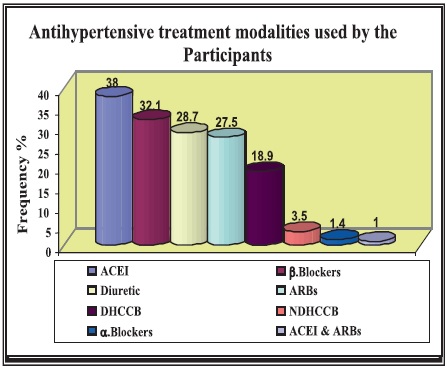 Figure 2. Antihypertensive treatment
Figure 2. Antihypertensive treatment
Overall, 34.6% of the patients with diabetes had albuminuria (29.3% had microalbuminuria and 5.3% had macroalbuminuria). No significant differences in gender and smoking were noted among patients with microalbuminuria and macroalbuminuria. The microalbuminuric patients were older compared with the normoalbuminuric group (p=0.001). There was an increase in frequency of microalbuminuria and macroalbuminuria in patients who had diabetes of more than 11 years duration. Mean SBP were significantly higher with microalbuminuria and macroalbuminuria compared with normoalbuminuria (p<0.0005). However, diastolic blood pressure was higher only in the normoalbuminuric patients (p<0.0005). Patients with microalbuminuria were predominantly obese and overweight (p=0.02). See Table 3.
The presence of hypertension, hypertriglyceridemia and hypercholesterolemia were significantly higher with both microalbuminuric and macroalbuminuric groups. HbA1c levels were significantly higher in all groups with abnormal albumin. The prevalence of diabetic retinopathy was significantly higher in microalbuminuric and macroalbuminuric patients, compared with those with normoalbuminuria (p<0.0005). See Table 4.
In the bivariate analysis, microalbuminuria was significantly associated with age, BMI, duration of diabetes, diabetic retinopathy, HbA1c, hypercholesterolemia, hypertriglyceridemia, SBP, and DBP. After using stepwise logistic regression analysis, the only variables remaining significantly associated with microalbuminuria were mean HbA1c, mean SBP and the presence of retinopathy. For each 1% increase in HbA1c, the odds of having microalbuminuria increased by 86%. Patients with mean SBP >130 mm Hg were found to be 1.85 times more likely to have microalbuminuria than those with SBP <130 mm Hg. Patients with mean DBP >80 mm Hg were found to be 1.47 times more likely to have microalbuminuria than those with DBP <80 mm Hg. The odds of having microalbuminuria for those who had diabetic retinopathy are 1.45 times more than those who did not have diabetic retinopathy. See Table 5.
Table 3. Prevalence of diabetic microalbuminuria and macroalbuminuria according to sociodemographic and anthropometric characteristics
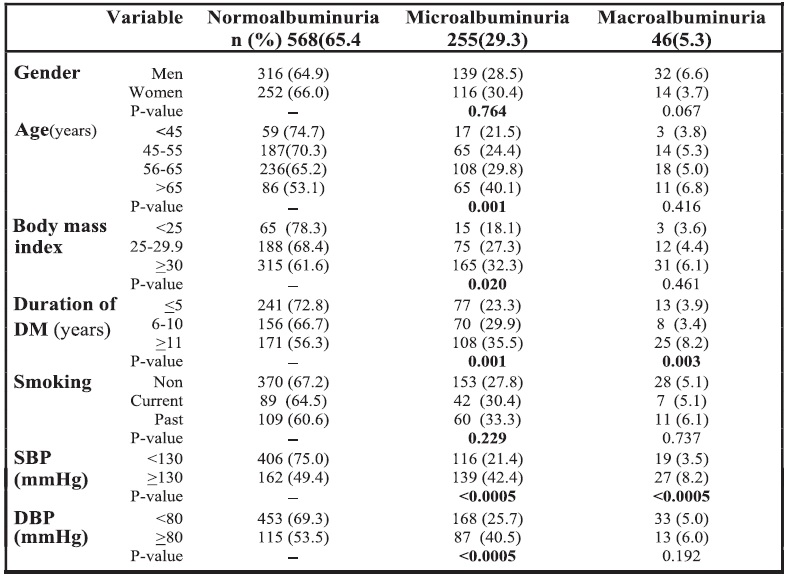
Table 4. Prevalence of diabetic microalbuminuria and macroalbuminuria according to clinical and biochemical characteristics
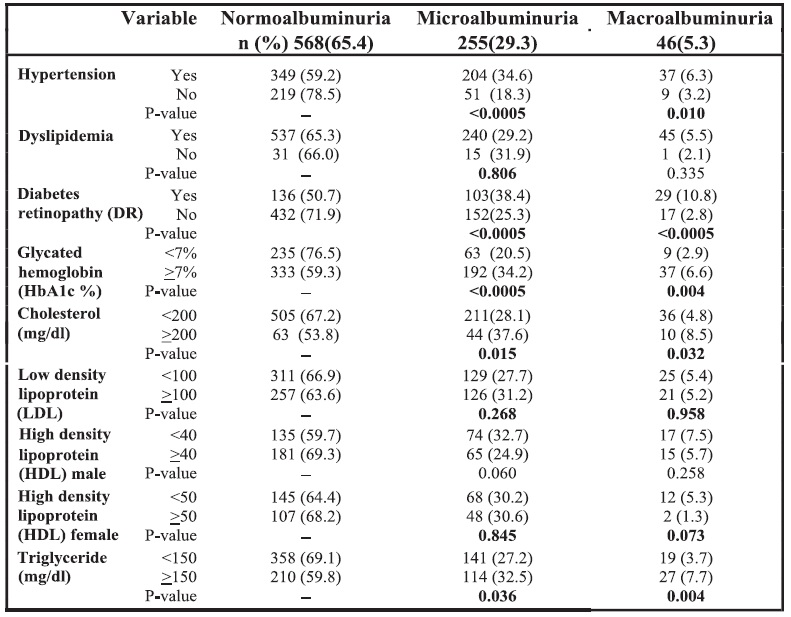
Table 5. Logistic regression analysis of factors associated with microalbuminuria
OR: Odds ratio; HbA1c: Glycated hemoglobin; SBP: Systolic blood pressure; DBP: Diastolic blood pressure
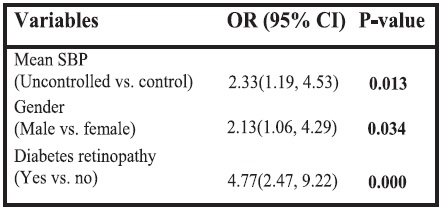
In the bivariate analysis, macroalbuminuria significantly associated with duration of diabetes, diabetes retinopathy, HbA1c, hypercholesterolemia, hypertriglyceridemia, SBP and DBP. After using stepwise logistic regression analysis, the only variables which remained significantly associated with macroalbuminuria were mean SBP, male gender and the presence of diabetic retinopathy. Patients with mean SBP >130 mm Hg were found to be 2.33 times more likely to have macroalbuminuria than those with SBP <130 mm Hg. Males were found to be 2.13 times more likely to have macroalbuminuria than females. Patients with diabetic retinopathy were found to be 4.77 times more likely to have macroalbuminuria than those without diabetic retinopathy.
Table 6. Logistic regression analysis of factors associated with macroalbuminuria
 OR: Odds Ratio. SBP: Systolic blood pressure
OR: Odds Ratio. SBP: Systolic blood pressure
This study measures the prevalence of the different stages of albuminuria in type 2 diabetic patients attending the NCDEG. As a national center receiving patients from all the country, either directly or referred from other clinics in Jordan, we believe that patients included in this study are representative of all diabetics in Jordan. Numerous studies have been carried out to determine the prevalence of albuminuria in patients with type 2 diabetes. These studies yielded rates between 17 and 58.6% for albuminuria19, 20, 21. Our study showed the prevalence rate at 34.6% which is in the median range. The marked variation in rate might be due to study design, sample selection, the method used for collecting urine, race and the age and gender structure of the population21. The prevalence of microalbuminuria and macroalbuminuria in our study was 29.3% and 5.3% respectively. The prevalence of microalbuminuria in this study is comparable to that found in the Hungarian study conducted by Molnár et al.22, which showed close prevalence (27.5%), but considerably higher prevalence of macroalbuminuria, 38.5% vs. 5.3% in our study. In Finland, a study conducted by Wirta et al. in recent-onset diabetic patients, showed almost the same prevalence as in our study: microalbuminuria and macroalbuminuria prevalence values of 29%, 4% vs. 29.3%, 5.3% among our patients23. However, a study carried out in the United Kingdom on NIDDM patients showed a lower prevalence of albuminuria (19%)24. Concerning Arab countries, studies in the Kingdom of Saudi Arabia25 and the United Arab Emirates26 showed a relatively higher rate of microalbuminuria, 41.3%, 61%, respectively, than that observed in our results. On the other hand, a study in Sultanate of Oman21, showed a nearly similar rate (27%).
Hyperglycemia is considered to be one of the important risk factors for the development and progression of diabetic retinopathy27. Several studies clearly demonstrated the benefits of improving glycemic control and decreasing HbA1c concentration in decreasing this complication rate12, 28. In our study, HbA1c showed a high correlation with microalbuminuria. On the other hand, macroalbuminuria showed no significant association with HbA1c. This finding is consistent with the results obtained by De Pablos et al. 29, but results must be considered with caution because of the cross-sectional design of the study.
Our study showed a significant association between systolic blood pressure and both microalbuminuria and
macroalbuminuria. Systolic blood pressure is identified as a risk factor for microalbuminuria and macroalbuminuria in many studies11, 19, 30. On the other hand, our data showed that diastolic blood pressure is significant with microalbuminuric groups only, which is consistent with the results of (CURES-45) study 30.
The association observed between albuminuria and the number of years since diagnosis was not significant in our study. However, this effect is difficult to determine in type 2 diabetes. A plausible explanation for this is the fact that non-insulin dependent diabetes may be silent for many years before clinical recognition, as well as the fact that these patients are subject to recall bias. This may explain why previous studies have reported both significant14, 29 and non-significant14, 22 associations between albumin excretion and duration of the disease.
The study data failed to show a positive association with smoking. The hazardous effect of smoking may be blurred by the substantially lower proportion of current smokers 15.9% in our patients sample than that in the United Kingdom study (31%)31. A plausible explanation for this association is that on diagnosis of nephropathy patients tend to quit smoking as part of a nephropathy interventional program, which is not the case in patients with normal albumin who see no reasons to quit smoking. In addition, no information was recorded regarding when they quit and therefore this finding justifies further research for this phenomenon. Contrary to our results, smoking was significantly associated with albuminuria22. However, Gall et al.14 and Varghese et al.27 did not observe any significant differences.
Surprisingly, gender was not statistically different when tested alone against the occurrence of microalbuminuria and macroalbuminuria. In the final regression model it shows significant correlation with male gender and with macroalbuminuria. Earlier studies have reported an increased association of microalbuminuria32 and macroalbuminuria20 with men compared with women. On the other hand, another study revealed that female gender was positively associated with microalbuminuria25. However, principally
because women have a lower creatinine excretion due to less muscle mass than men, it can be problematic when using the albumin-creatinine ratio to compare prevalence across genders. Thus some investigators often use a lower threshold for men than women25. It remains to be seen whether the difference in albumin excretion between men and women has significance for long-term risk of chronic disease or merely reflects the increased size of men compared to women.
Age was not identified as a risk factor in our study, which is in agreement with the findings of several other investigators who reported no significant correlation between age and microalbuinuria11, 20, 28 and macroalbuminuria27. In contrast, some other studies showed that age is highly predictive of microalbuminuria14, 32 and macroalbuminuria40. This could be attributed to the fact that our study was crosssectional and as such its results cannot be used to correctly trace or establish the role of age in the development of microalbuminuria and macroalbuminuria in the sample patients.
It is of interest that body mass index did not correlate significantly with either microalbuminuria or macroalbuminuria in the logistic regression analysis. These results are in agreement with previously published data21, 27, 28, 37. On the other hand, a cross-sectional study by Cederholm et al. reported that the prevalence of microalbuminuria was positively associated with BMI32. Furthermore, one study reported that patients with BMI <18kg/m² were more likely to have proteinuria33. This issue is still unresolved and as such needs further investigation.
According to serum lipid, our study showed no association between serum lipids and either microalbuminuria or macroalbuminuria and is consistent with a number of other studies26, 27. This study showed that a high percentage of patients are on antilipidemic drugs. This might have afforded a degree of protection against diabetic kidney disease, as evidenced by several studies that lipid reduction by antilipidemic agents might decrease proteinuria in diabetic patients21, 26. While there are a number of studies showing a significant association between serum lipid and either microalbuminuria or macroalbuminuria14, 22, this result remains a debatable issue concerning albuminuria and needs to be addressed by future longitudinal studies.
In this study we found that the number of patients with microalbuminuria, macroalbuminuria and retinopathy was statistically significant which was a finding supported by other studies17, 30. Moreover, our results revealed that the prevalence of DR in microalbuminuria patients was higher than that in macroalbuminuria patients (38.4% vs.10.8%), which confirmed that even patients who have microalbuminuria were at high risk of developing retinopathy11. It was surprising to note that in our 46 patients with macroalbuminuria, 29 (63%) of them had DR, whereas diabetic retinopathy was absent in 17 (37%) of the 46 patients with macroalbuminuria. In a different study, analysis of type 2 diabetics with proteinuria found that approximately 30% did not have diabetic nephropathy on renal biopsy among those without retinopathy34. Type 2 diabetics with marked proteinuria and retinopathy are most likely to have DNP, whereas in those without retinopathy it may be caused by disease other than diabetic glomerulopathy (hypertension, atherosclerosis, chronic glomerulonephritis, chronic pylonephritis)22.
This is a center-based study which explored the prevalence of albuminuria in patients attending the NCDEG in Amman, Jordan, a tertiary center with referrals from all over Jordan.
Our study showed:
In conclusion, given the large numbers of persons with diabetes in Jordan2, the number of diabetic patients with microalbuminuria and macroalbuminuria is significant. The large pool of those with microalbuminuria suggests that there could be large increases in those with macroalbuminuria over time9. Most patients with macroalbuminuria eventually reach end-stage renal disease (ESRD)9. Although the cost of hemodialysis in Jordan is relatively low (US$ 17,385/patient/year), the burden is huge in a country with limited resources10. There is thus a degree of urgency for implementing early and universal screening programs for microalbuminuria in type 2 diabetic patients. Through cardiovascular risk reduction, lowering blood pressure, renin-angiotensin system blockade and glycemic control, such early and targeted intervention could significantly reduce the occurrence of renal complications. In addition, early referral to nephrologists should be mandatory, especially for those with macroalbuminuria in the absence of retinopathy, for further evaluation of “non-diabetic” glomerulopathies. Since DR is a frequent occurrence in the presence of diabetic nephropathy, it is particularly important to investigate all patients with albuminuria for DR by an experienced ophthalmologist. This should be according to ADA guidelines which recommended annual measurement of serum creatinine for the estimation of GFR in addition to measurement of urine albumin excretion15. Further research is recommended to evaluate these guidelines, especially in whose patients who manifest with decreased GFR in the absence of increased urine albumin excretion.