
Journal of the Bahrain Medical Society
Year 2018, Volume 30, Issue 1, Pages 5-8
https://doi.org/10.26715/jbms.1_01032018Nandan Shanbhag1
1Internal Medicine, Bahrain Specialist Hospital, Rd No 2447, Manama 10588, Kingdom of Bahrain.
*Corresponding author:
Nandan Shanbhag, Internal Medicine, Bahrain Specialist Hospital, Rd No 2447, Manama 10588, Kingdom of Bahrain; Email: Onco@drnandan.com
Received date: January 15, 2018; Accepted date: March 01, 2018; Published date: March 20, 2018

A 69-year-old British gentleman with a good performance status, who runs a charity for animals (important), presented to the Gastroenterology outpatient department at the Bahrain Specialist Hospital (June 2016) with complaints of vague abdominal discomfort, bloating sensation, tiredness, and a recent change in the bowel habits. Endoscopy revealed a rectosigmoid colon mass, and the biopsy confirmed an invasive adenocarcinoma. The elderly man received six cycles of combination chemotherapy with oxaliplatin and capecitabine (until January 2017); however, the disease progressed, and he had liver metastases. A total mesenteric excision along with metastasectomy of the liver lesions was performed (February 2017); and a follow-up positron emission tomography–computed tomography (PET-CT) image showed a progressive disease with multiple lesions in both lobes of the liver as well as local recurrence. He was depressed and wanted to know further options; and so, was started on injection panitumumab 6 mg/kg administered every 21 days. After four doses of the therapy, PET-CT showed complete resolution of the local recurrence and partial resolution of the liver lesions. The patient, then resumed his activities with a good performance status and the therapy with panitumumab will be continued until either, disease progression or severe side effects are observed. The case illustrates the effectiveness of panitumumab in metastatic colorectal carcinoma, which has progressed on standard chemotherapy regimen.
Keywords: Metastatic colorectal cancer (mCRC), response rate therapy, biotherapy, epithelial growth factor receptor, panitumumab.
The recent advances in the treatment of metastatic colorectal cancer (mCRC) and the irrefutable link between respectability and overall survival has led to the practice of ‘response rate therapy’.1 The most common site of metastases in colorectal malignancy is liver, and operability at presentation determines the overall survival, with a survival rate of more than two years. This was well demonstrated in a prebiologics era trial, with a median survival of 21 months.2 Median survival in mCRC in the prebiologics era was observed with a combination chemotherapy, including fluoropyrimidines, irinotecan, and oxaliplatin—either used as combination of doublets or sequentially.3
The discovery of epidermal growth factor receptor (EGFR) by Stanley Cohen in the early 1980s opened the door for a new form of therapy that used the drugs to target these specific receptors, often on the tumor cells.4 The era of RAt Sarcoma (RAS) mutation testing in colorectal carcinomas, most specifically mutations of the KRAS codon 2 and exon 12 and 13 observed in about 40% of the patients with colon cancers, concluded that these patients are resistant to EGFR drugs.5
The endpoint of any treatment with a palliative intent is quality of life and treatment response. The response assessment in solid tumors is performed by modified response evaluation criteria in solid tumors (RECIST)1.1 criteria.6 This case report illustrates the response to a novel targeted agent, panitumumab—a human monoclonal antibody EGFR inhibitor—in a patient with recurrent metastatic colorectal carcinoma.
A 69-year-old British gentleman with a performance status of Eastern Cooperative Oncology Group (ECOG)-1, who operates a charity for animals (important), first consulted to the Gastroenterology outpatient department in June 2016 with complaints of vague abdominal discomfort, bloating sensation, tiredness, and a recent change in the bowel habits. He was evaluated by the team; and a biopsy confirmed an invasive adenocarcinoma of the colorectum region along with liver metastases.
He has hypertension and is being treated with a lowdose beta-blocker. He quit smoking after 30 pack years. Alcohol consumption was about 30 ml per day. He has a history of rectal cancers in the family, with two brothers diagnosed and treated for the same. After discussion with the patient, a decision was made by the multidisciplinary tumor board to initiate chemotherapy with a palliative intent. He received six cycles of combination chemotherapy with CAPOX, consisting of oral capecitabine (850 mg/m2 for 14 days) and intravenous oxaliplatin (130 mg/m2 every 21 days), which showed progressive disease at the end of the cycles. Surgical intervention was sought and a total mesenteric excision along with metastasectomy of the liver lesions was performed. Postoperative computed tomography (CT) scan of the abdomen revealed residual lesions in the liver with the appearance of newer lesions (Figure 1).
The patient had a repeat consult in the Oncology clinic in June 2017 with complaints of loss of appetite, loss of weight, and inanition. On examination, the patient appeared agitated, noncooperative, poorly built, and nourished with a decline in the performance status to ECOG-2. He had a colostomy bag in situ with a liver span of 17×18 cm, palpable 5 cm below the costal margin with no other palpable mass in the abdomen. A scar was noted on the right tibia and fibula, which signified a traumatic fracture in the past.
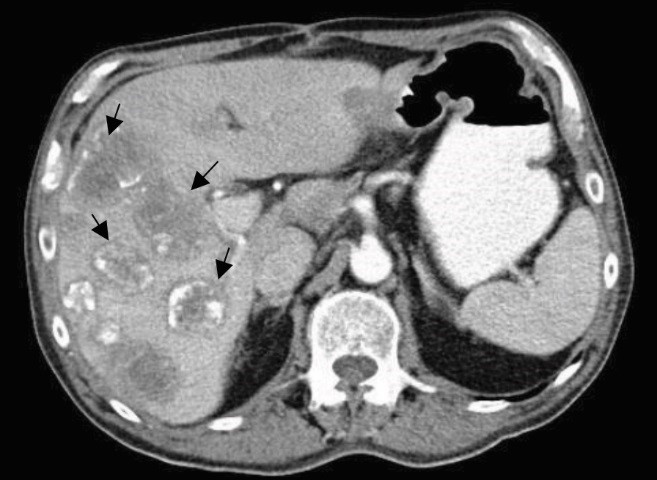 Figure 1: Computed tomography (CT) image (March 2017) showing evidence of surgery and multiple liver lesions (arrows)
Figure 1: Computed tomography (CT) image (March 2017) showing evidence of surgery and multiple liver lesions (arrows)
A positron emission tomography–computed tomography (PET-CT) scan was requested to apprehend the status of the disease outside the liver, and review of the histopathology report of the previous palliative surgery revealed a positivist for EGFR with a wild type RAS gene. The repeat whole-body PET-CT revealed multiple [18F] fluorodeoxyglucose (FDG) avid liver lesions in both lobes of the liver with FDG uptake in the rectosigmoid region; multiple para-aortic region; external, internal, and common iliac region; and intestinal nodes (Figures 2, 3, 4, and 5).
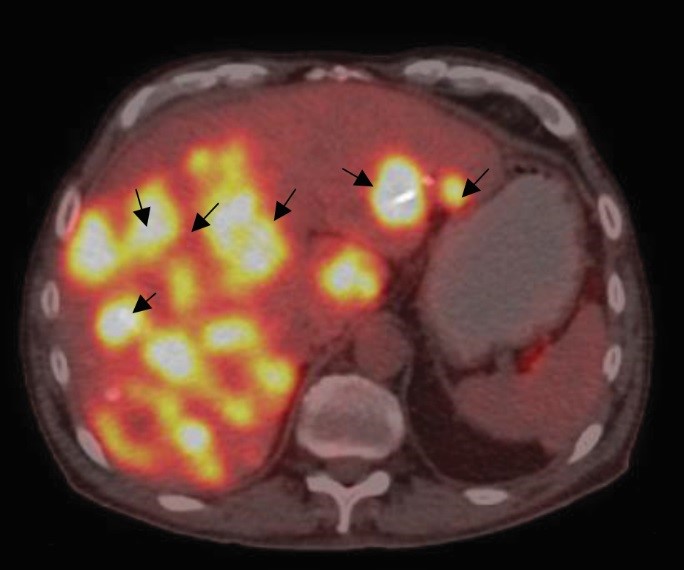
Figure 2: Base positron emission tomography–computed tomography (PET-CT) image (June 2017) showing multiple liver lesions, prior to epidermal growth factor receptor therapy (arrows)
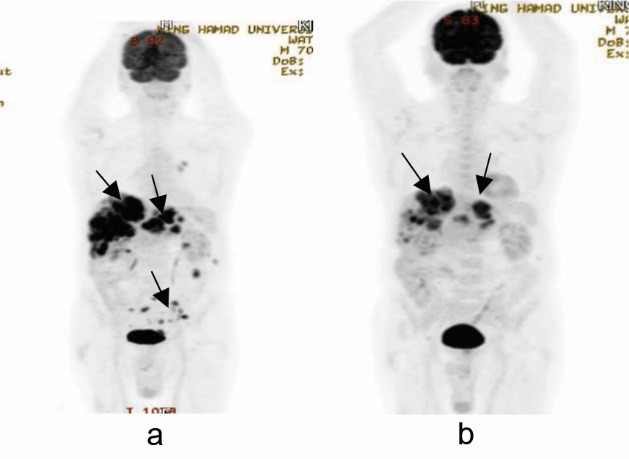
Figure 3: Comparative whole-body PET images showing (a) pre (June 2017) and (b) post (Oct 2017) epidermal growth factor receptor inhibitor therapy (arrows)
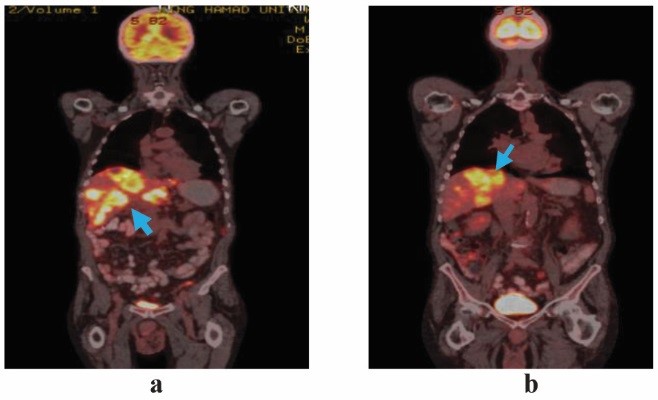
Figure 4: Comparative coronal section of wholebody fused PET-CT images showing (a) pre (June 2017) and (b) post (Oct 2017) epidermal growth factor receptor inhibitor therapy (arrows)
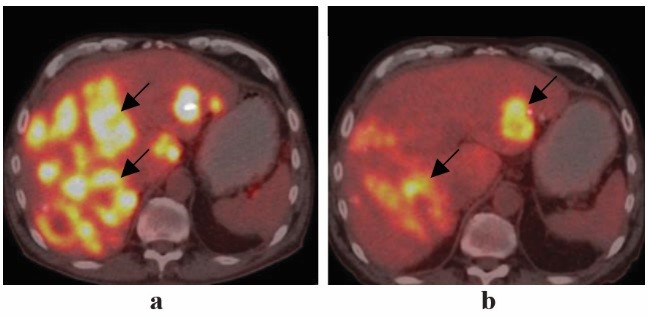
Figure 5: Comparative axial section PET-CT images showing (a) pre (June 2017) and (b) post (Oct 2017) epidermal growth factor receptor inhibitor therapy (arrows)
After a detailed discussion with the patient and given the naivety of the patient to EGFR therapy, it was decided to administer four doses of EGFR inhibitor and assess tumor response. The drug chosen was injection panitumumab 6 mg/kg body weight administered every 21 days.
The patient tolerated the first cycle notably well, and after 14 days developed grade 2 papulopustular rash predominantly over the face, back, and arms. The rash completely subsided with treatment and the patient had only grade 1 rash after each cycle, which subsided with the application of an alcoholfree moisturizer containing 2% clindamycin used twice daily for seven days.
A repeat PET-CT after four doses of panitumumab showed partial response to the therapy according to the RECIST 1.16 (Figures 3, 4, and 5). The liver lesions reduced by 30% as compared to the pretherapy scan, and there were no lesions in the rectosigmoid region and nodal stations that were FDG avid or detected in the CT scan.
The patient showed remarkable improvement in activities of daily living with the performance status returning to ECOG-0 and him being able to do all his activities without any support, including driving. He gained weight of about half a kilo every cycle.
Given the good response to the therapy, the patient requested a colostomy reversal and after discussing with the surgeon, a decision was made to continue panitumumab therapy for three more cycles and assess the probability of reverse colostomy.
EGFR is a protein that is found in the skin, colon, and the lungs. It is a tyrosine kinase receptor and was first discovered in the epidermis of the skin, hence the name. Mutation of these receptors occurs only in a tumor and not in the normal tissue, and this mutation drives cancer cells to grow. All mCRCs must be tested for RAS mutations as the tyrosine kinase inhibitors do not act on a mutated EGFR and 40% of the mCRCs are known to harbor this mutation.7 Panitumumab is an EGFR inhibitor which acts by preventing the dimerization of the receptor, thereby preventing auto-phosphorylation leading to inactivation of the RAS pathway. This leads to inhibition of the growth factor release leading to promotion of apoptosis, inhibition of angiogenesis, proliferation, and metastases8 (Figure 6).
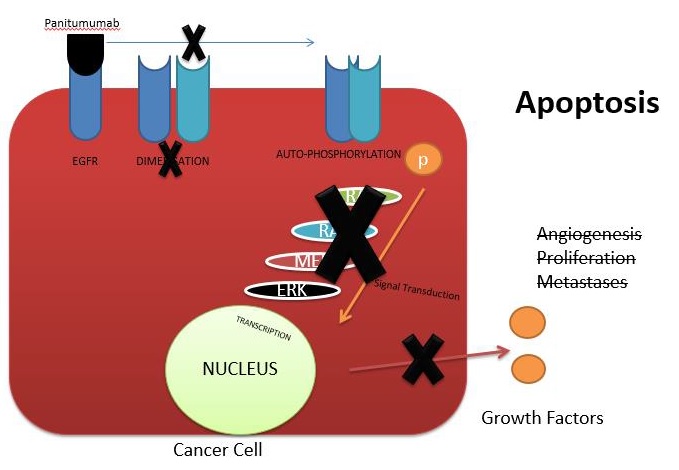
Figure 6: Mechanism of action of panitumumab
Panitumumab is indicated as a single agent for the treatment of metastatic colorectal carcinoma with disease progression on or following fluoropyrimidine, oxaliplatin, and irinotecan chemotherapy regimens.9 A common side effect is the papulopustular rash, which may progress to crusting and is treated with non-alcoholic moisturizers as well as steroid-based creams. This skin rash is an important side effect as evidence suggests that the appearance and severity of the skin rash are correlated with objective tumor response and overall survival in metastatic colorectal carcinoma.10
This case demonstrates the effectiveness of the targeted therapy in an advanced malignancy, while also providing better quality of life. Majority of guidelines suggest the use of targeted therapy in conjunction with chemotherapy and not as upfront single-agent therapy. Whether these targeted drugs can be suggested as upfront single agents for advanced metastatic colorectal carcinomas requires further study and a larger case series.