
Journal of the Bahrain Medical Society
Year 2014, Volume 25, Issue 1, Pages 42-46
https://doi.org/10.26715/jbms.p25_9Hasan M. Isa,* Lina F. Al Ali,** Afaf M. Mohamed,*** Rawia M. Hamad,****
*Consultant Pediatric Gastroenterologist, Salmaniya Medical Complex, Kingdom of Bahrain
**Pediatric Senior Resident, Salmaniya Medical Complex, Kingdom of Bahrain
***Family Physician, NBB Dair Health Centre, Kingdom of Bahrain
****Pathologist, Salmaniya Medical Complex, Kingdom of Bahrain
Correspondence to: halfaraj@hotmail.com

Giant cell hepatitis (GCH) with autoimmune hemolytic anemia (AIHA) is a rare disease occurring early in childhood. The exact cause of this illness is unknown and is assumed to be an autoimmune disorder. This disease often has a fatal outcome due to poor response to immunosuppression. In this paper, we report an 11-month-old male infant who presented initially with Coomb’s positive autoimmune hemolytic anemia followed by histology-proven GCH and rapid progression to fulminant hepatic failure and death within two months from the onset of the disease despite aggressive immunosuppression. In addition, we review the available literature about the clinical presentation, diagnosis and therapeutic options of this rare disease.
Keywords: giant cell hepatitis; autoimmune hemolytic anemia
Giant cell hepatitis (GCH) is commonly found in infants with neonatal cholestasis.1 It is also known as post-infantile or syncytial giant cell hepatitis, and is primarily seen in the first three months of life.2 Although GCH does not indicate a specific cause or disease, it has been associated with various conditions like viral infections, drug induced hepatotoxicity and autoimmune disorders.3
GCH with autoimmune hemolytic anemia (AIHA) is a rare disease occurring early in childhood.1, 4, 5 Only 27 cases have been reported since the disease’s description in 1981.6 It is a fatal disease4, 7 different from other causes of giant cell transformation.5 The pathogenesis of the disease is unknown.1, 8 Some studies suggested that the cause is autoimmune due to the association with Coomb’s positive autoimmune hemolytic anemia and the response to immunosuppressive therapy.1, 4, 7 GCH with AIHA follows a progressive, lethal course requiring multiple immunosuppressive therapy.4, 7, 9 The liver disease progresses rapidly to hepatic failure, cirrhosis and ultimately death.10 However, to date no associated autoimmune markers have been identified in affected patients.7
Several treatment modalities have been tried on patients with this condition. Immunosuppression using steroid and azathioprine was found to be beneficial.1, 10 Some cases which fail to respond to steroid and azathioprine have benefited from rituximab, a CD20 monoclonal antibody.3 Splenectomy and liver transplant were also tried in those patients, however splenectomy carries a risk of death from infection and the liver transplant showed a high rate of recurrence of severe liver disease in the first month following the transplant.9, 11 This is the first reported patient with this condition in the Kingdom of Bahrain.
This is a case of an 11-month-old Bahraini male, a product of full term normal vaginal delivery with birth weight of 2.5 kg, who was referred to Salmaniya Medical Complex (SMC) for investigation and management of hemolytic anemia. The infant had no ante- or post-natal complications and he was the youngest of 10 healthy siblings of nonconsanguineous parents. There was no significant family history of any immune or hepatic diseases. Two months prior to his referral, he presented with high-grade fever of 40° C, hypoactivity and pallor of one day duration. Physical examination revealed a pallor and hepatosplenomegaly. His Hb was 4 g/dl, thus packed red blood cell (PRBC) transfusion was given, along with intravenous immunoglobulin (IVIG) and methylprednisolone due to suspicion of AIHA, as his direct Coomb’s test (DCT) and indirect Coomb’s test (ICT) were positive. He was discharged after one week with Hb of 9 g/dl. Two weeks later, he became hypoactive and pale and his Hb dropped to 7 g/dl, thus he received the second PRBC transfusion and was discharged after 2 days with Hb of 11 g/dl. One week later, the third transfusion was given because his Hb was 4 g/dl. After the third transfusion, he was irritable, vomiting, passing dark-colored urine and deeply jaundiced. The patient received further IVIG for two days followed by oral prednisolone 5 mg TDS and was referred to our hospital.
Table 1. Results of laboratory tests from the day of admission to the day of death
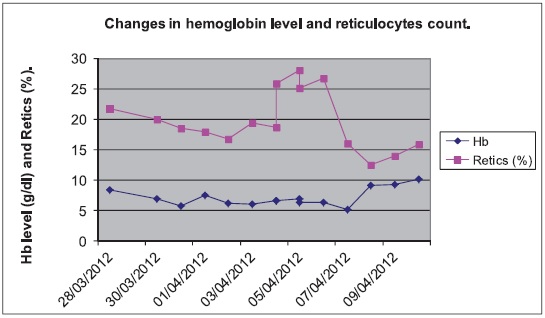
Figure 1. Hemoglobin level and reticulocyte count
On presentation to SMC, the patient was pale, icteric and febrile with a temperature of 38° C. His pulse rate was 165, respiratory rate 50 cycles/minute, blood pressure 111/59 mm Hg and oxygen saturation was 99%. His weight was 8 kgs (between 3rd and 15th centile), height 73 cm (between 15th and 50th centile); head circumference 43 cm (on 3rd centile). There was firm hepatomegaly 4 cm below the costal margin and splenomegaly 6 cm below the costal margin. The laboratory tests were repeated and the results are shown in Table 1 and Figure 1.
The patient’s laboratory investigation showed positive DCT 4+ and ICT 4+ but antinuclear antibodies (ANA), antismooth muscles (anti-SM) antibodies and anti-liver kidney microsomal (anti-LKM) antibodies were all negative. He had normal glucose-6-phosphate dehydrogenase activity. Hepatitis A, B, C and E were all negative. CMV and toxoplasma serology were negative. VDRL was non-reactive while EBV serology showed evidence of past infection. Blood, urine and stool culture were negatives. Abdominal ultrasound showed splenomegaly, hepatomegaly with homogenous echo texture and no evidence of intra- or extra-hepatic bile duct dilatation. Gallbladder, kidneys and pancreas were normal. The patient was treated with cefotaxime, ursodeoxycholic acid, vitamin E orally, intravenous vitamin K, one dose of IVIG 1 g/kg and ethylprednisolone 1 mg/kg.
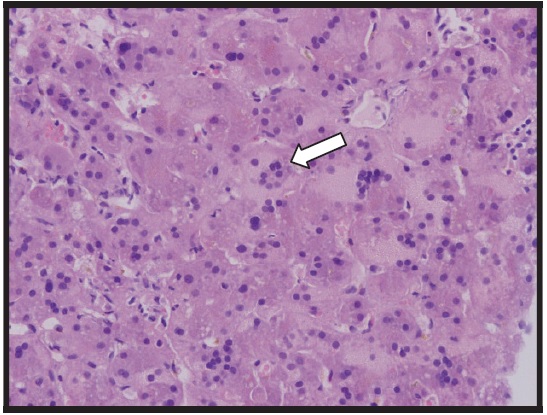
Figure 2. Liver biopsy, hematoxylin and eosin stain (20×) showing giant cells
On day 5, the patient’s clinical condition deteriorated. He developed fulminant hepatic failure with grade 2 hepatic encephalopathy so he was shifted to PICU. His liver function tests were worsening so a higher dose of methylprednisolone, 30 mg/kg/day, was given. One day later, an ultrasound-guided liver biopsy was performed which showed evidence of giant cell hepatitis (GCH) with excess of iron deposition.
The patient was diagnosed to have GCH and Coomb’s positive AIHA and started on Rituximab 10 mg/kg IV infusion while methylprednisolone was changed to prednisolone 2 mg/kg/day. Desferoxamine slow infusion 4 g/day was started because of high serum ferritin.
The patient did not improve; he continued to have encephalopathy and coagulopathy. In addition, he developed coffee ground vomitus, massive ascites and his Hb dropped again to 6.4 g/dl. PRBC transfusion was given and the prednisolone dose was increased to 2.5 mg/kg/day.
On day 12, the patient developed hypotension so normal saline bolus and dopamine were given. And one day
later, he had mid-dilated pupils with sluggish reaction to light. Brain CT scan showed diffused cerebral dema and early transtentorial herniation. The patient was intubated, hyperventilated and started on manitol. On day 14, his clinical condition deteriorated more with worsening sensorium. CT brain was repeated and it showed increased brain edema and transtentorial herniation with evidence of anoxic encephalopathy. On day 15, he had convulsion and he required resuscitation twice with maximum support. The patient was declared dead with a total hospital stay of 15 days.
GCH-AIHA is a disease of infancy and is not reported in late adolescence or adulthood.3, 10, 11 Disease onset, in most of the cases, was between 4 and 24 months of age and the youngest reported case was at three months.3 Our patient presented at the age of nine months. There is only one reported case in later life, presenting at the age of 18 years, which is unusual.11
The cases of GCH-AIHA reported in the literature are quite uniform in their clinical presentation, clinical progression, biochemical and histologic findings, as well as their response to therapy, and poor prognosis.1 Similar to our patient, the patients usually presented with symptoms of acute hemolysis (fever, pallor and hepatosplenomegaly) and Coomb’s positive hemolytic anemia. Anemia frequently precedes the development of hepatitis by months to years, and usually these patients are initially diagnosed with isolated AIHA.10 The progress of anemia may be independent of the evolution of hepatitis.6
GCH may have a similar presentation to viral hepatitis with comparable signs, symptoms, and laboratory findings, but more commonly has an insidious onset over several weeks or months.10 It might occur within 1 week to 15 months (usually 1-2 months) after the diagnosis of AIHA.11 Liver disease progresses quickly to cirrhosis and hepatic failure.11 The clinical course is aggressive1, 12 and usually fatal due to poor response to immunosuppression.1, 3, 10 Hepatic failure, unexplained encephalopathy, severe seizure disorder, and severe infection are universally the causes of death.1, 12
Coomb’s positive autoimmune hemolytic anemia (AIHA) is rarely associated with GCH in pediatric patients.3, 4, 7, 10, 11, 12, 13 The exact causes and the pathogenesis are not well understood but is assumed to be autoimmune.3, 5, 7, 10 Some studies suggested that GCH-AIHA is a disease of generalized immune dysfunction.6, 7 There are several points that could support the theory of immune dysfunction like the family history of immune diseases: the presence of autoantibodies or immune thrombocytopenia in some children, a Coomb’s test of the IgG + C type, the efficacy of immunosuppressive therapy, and the occurrence of relapses when treatment doses are reduced.1, 6, 11 In addition, failure of liver transplantation in GCH-AIHA is high in comparison to other types of GCH seen in children which also supports the hypothesis of an underlying immune dysregulation.3 Some studies suggested a relation between certain viruses and this disease. An interaction between host immune system and virus antigen might play an important role in triggering immunopathologic phenomena.14 Urganci et al.15 suggested that autoimmune hepatitis and AIHA were induced by recurrent infections. Although certain viruses can lead to giant cell hepatitis, none have been recognized in this disorder.11 Hepatitis C viral infection is the main virus reported with AIHA, but cases of viral hepatitis B and A are also recorded.14, 15 In a case report, GCH-AIHA developed after varicella infection.13
GCH-AIHA is characterized by multiple diagnostic difficulties.4 Positive DCT is the only sign of autoimmunity in patients with this disease.10 In GCH-AIHA, unlike autoimmune hepatitis, autoantibodies are usually negative.12 Specific autoimmune markers in this particular combination of GCH and AIHA are yet to be defined.3 Episodic tests of autoimmune markers are necessary as they might develop later.3 This disorder is considered to be a distinct syndrome and the serological tests for anti-nuclear, anti-smooth muscle, anti-liver kidney microsomal (LKM- 1), and anti-mitochondrial antibodies are negative.10 A case report of one patient showed anti- LKM-1 antibody positive but it was in low titers (1:10).10 Another case report showed multiple immune phenomena in a patient with GCH-AIHA were antinuclear antibodies, anti-RNP, anti-SS-A, and anti-SS-B autoantibodies.1 Until now there has not been a recognized case of anti-mitochondrial antibodies.7
Our patient had Coomb’s tests and liver biopsy positive but all other autoantibodies were negative. Patients with AIHA and abnormal liver function should undergo early and aggressive evaluation, including autoimmune markers tests and a liver biopsy.7
In cases of GCH-AIHA, the reported liver histology was strikingly similar in all patients and was characterized by distortion of lobular architecture because of diffused giant cell transformation and a variable degree of parenchymal collapse.1, 10 The histological features characteristic of autoimmune hepatitis are missing in patients with GCHAIHA, which are periportal hepatocellular necrosis along with bridging fibrosis and multilobular necrosis.7, 12 Giant cell transformation is a pathologic finding that can be seen in liver biopsy specimens performed during the neonatal period for neonatal hepatitis evaluation.7 The origin and mechanisms of giant cell transformation have not been clearly established.1, 8, 11 Giant cell transformation is clearly uncommon after the neonatal period.1 Post-infantile GCH is a descriptive diagnosis of a unique pattern of reaction to several different insults secondary to multiple etiologies.1 This nonspecific hepatic response is commonly present in infants with liver disorders including neonatal hepatitis, biliary atresia, and chronic active hepatitis.7 In addition, hemophagocytosis within the spleen, lymph nodes, and bone marrow was also reported in a child with GCHAIHA. 1 Hemophagocytosis may be part of the disease’s clinical spectrum, a late complication of the disease itself, or due to the massive immunosuppressive therapy.1
Management of patients with GCH-AIHA is a challenge.10 The response of these children to immunosuppressive drugs is variable.10 As with the present patient, GCH-AIHA has partial response to steroids with a need for high doses of numerous immunosuppressive drugs and lack of persistent response.4, 16 Early initiation of steroids has been shown to have a beneficial effect for both liver function and AIHA.10, 13 Steroids can significantly reduce the mortality in the early active phase.10 In most patients there is an initial response to immunosuppressive treatment, but relapses occur commonly.1 The high initial dose should be continued for as long as possible until serum aminotransferase activity has returned to normal, because early relapses may occur.6
Extreme caution should be exercised when reducing steroid doses.6 Any reduction of the dose of steroids initially given to treat AIHA should be gradual because the liver condition might progress to liver failure if the dose is lowered too fast.6 Moreover, it has been reported that hepatitis relapses are difficult to manage and may be controlled only by increasing the steroid dosage or adding cyclosporine to the initial regimen.6 Sometimes, GCH is refractory to drug therapy.6 Patients who do not respond to steroids may require other immunosuppressive drugs like cyclophosphamide or azathioprine.10 Because our patient was rapidly deteriorating, he did not receive azathioprine as it needs a long time to act. Immunosuppression with steroid and azathioprine is required for maintaining remission.1 Usually, the hemolytic anemia is controlled well after the initial episode of treatment.1, 11 Patients who have GCH-AIHA and who are unresponsive to immunosuppressive therapy should have electron microscopy evaluation of their liver and the diagnosis of syncytial GCH should be considered.7 In a case report, one patient was successfully weaned from immunosuppressive regimen and remains in remission.7 In that patient, early implementation of prednisolone therapy and a slow immunosuppressive taper with documented giant cell hepatitis may have contributed to the therapeutic success.7 GCH-AIHA has shown to be a progressive and often fatal disease process with a survival rate, without orthotopic liver transplantation (OLT), of approximately 50%.4 Only the combination of a long-term tapering dose of steroid and cyclophosphamide was found to be effective, and it could avoid a splenectomy which had already been planned because of the life-threatening anemia.4 The clear cut relationship between cyclophosphamide therapy and clinical/biochemical changes makes the response to this drug unlikely to be coincidental to a natural course of the disease.4 Because cyclophosphamide benefits might exceed its possible long-term risks, it has been suggested that this therapy deserves consideration when facing the emergency of drug resistance aggravating hemolytic crisis and/or liver disease in a child with GCH-AIHA.4 The determinant of response to immunosuppressive therapy has not been clearly established because of the limited number of patients.11
Conventional immunosuppressive therapies, such as steroid, azathioprine, cyclophosphamide, cyclosporine and mycophenonlate mofetil (MMF), are associated with high toxicity and often produce partial or short lasting remission.11, 12 Rituximab should be considered for GCH patients who do not respond to conventional immunosuppression.3 Rituximab, an anti-CD20 monoclonal antibody, prevents further antibody production through inhibition of B cell proliferation.3, 13 Alemtuzumab is another immunosuppressive drug that might be used and can lead to long-term remission of GCH-AIHA. One case was treated with alemtuzumab and has achieved a complete, long-lasting remission after temporary CD52+ cell depletion, with discontinuation of steroid and sustained normalization of liver function and histology.12
Liver transplantation (OLT) may be a lifesaving option in the late stage of the disease.11 Information about the effect of OLT in patients with GCH-AIHA is scant because only a few patients have undergone OLT after a trial of immunosuppressive therapy.11 Three of four patients died and all of the three had recurrence of GCH in the transplanted liver within 4 weeks after OLT.11 One patient was reported to have no recurrence after OLT. This was attributed to the use of MMF for maintenance immunosuppression.11 MMF was successful for the treatment of established rejection, primary rejection prophylaxis and toxicity secondary to CsA after pediatric OLT.11 OLT must be considered when signs of hepatic failure occur early or during a relapse and are refractory to immunosuppressive therapy.6 In contrast with other types of GCH, OLT in GCH-AIHA appears to be associated with a poor prognosis7, 8 and must be considered as a distinct entity.5
GCH with AIHA is a rare entity almost limited to young children. This disease should be considered in the differential diagnosis of fulminant hepatic failure in children. It carries a very poor prognosis. We strongly recommend that any patient with AIHA and abnormal liver function should undergo early and aggressive evaluation including a liver biopsy and autoimmune markers tests along with early implementation of immunosuppressive therapy.